More than 90% of prescriptions filled in the U.S. are for generic drugs. Yet, many patients still hesitate to take them. Why? Because they don’t understand what generics really are. A pill that looks different, costs less, and has a different name can feel suspicious-even if it’s exactly the same medicine. That’s where infographics about generics come in. They turn confusing science into clear pictures that patients can actually use.
Why Do Patients Doubt Generic Medications?
People don’t mistrust generics because they’re anti-savings. They mistrust them because they’re misinformed. Many believe that if a drug looks different or costs less, it must be weaker. Some think the FDA lets cheaper versions slip through without proper testing. Others worry about inactive ingredients-dyes, fillers, or coatings-that change the pill’s color or shape but don’t affect how the medicine works. A 2021 FDA survey found that 43% of patients were unsure if generic drugs worked as well as brand-name ones. That’s not just a knowledge gap. It’s a safety risk. When patients refuse generics, they skip doses, switch back to pricier brands, or stop treatment entirely. The result? Higher hospitalization rates, worse health outcomes, and billions in avoidable costs. This is where visual tools make a difference. Text-heavy brochures get ignored. Verbal explanations get forgotten. But a well-designed infographic? It sticks.How FDA Infographics Explain Generic Equivalence
The U.S. Food and Drug Administration (FDA) leads the way with a suite of free, science-backed infographics. Their most powerful one, titled “What Makes a Generic the Same as a Brand-Name Drug?”, uses simple visuals to show how generics work. It compares the active ingredient in a brand-name drug to its generic version using side-by-side molecular diagrams. Both are identical. Then it shows dissolution graphs-how fast the pill breaks down in the body. The curves overlap perfectly. That’s not marketing. That’s data. In FDA testing, 89% of patients correctly understood this visual proof of bioequivalence. Another infographic breaks down the approval process. It walks viewers through five steps: chemical analysis, bioequivalence testing, manufacturing inspection, quality control, and post-market monitoring. Each step has a simple icon: a flask, a graph, a factory, a checklist, and a magnifying glass. No jargon. No fine print. Just clear steps anyone can follow. These aren’t just pretty pictures. They’re validated. Before release, each infographic is tested on at least 30 real patients from diverse backgrounds. Comprehension scores average 87%. That’s higher than most doctor-patient conversations.What Makes These Infographics Different From Other Patient Materials?
Not all patient education tools are created equal. Some come from pharmacies, others from nonprofits or drug companies. But the FDA’s generics infographics stand out in three key ways. First, they’re standardized. Every graphic uses the same colors, fonts, and layout. That consistency builds trust. Patients know they’re getting the same reliable message no matter where they see it. Second, they’re accessible. All materials meet WCAG 2.1 AA standards for accessibility. High-contrast colors, alt text for screen readers, and plain language (tested at an 8th-grade reading level) mean people with low vision, learning differences, or limited English can still understand them. The FDA even offers all materials in Spanish under the title “Medicamentos Genéricos.” Third, they’re backed by real-world results. At Kaiser Permanente clinics, pharmacists who used these infographics saw a 63% drop in patient refusals of generic substitutions. One pharmacist posted on Reddit: “I’ve printed this and keep it behind the counter-cuts counseling time in half for generic questions.” Compare that to other resources. The GTMRx Institute offers great clinical insights but lacks multilingual support. BeMedWise includes handy medication logs but barely covers generics. Only the FDA’s materials combine regulatory authority, universal design, and proven impact.Where Are These Infographics Used-and How?
These tools aren’t sitting on a government website gathering dust. They’re in use everywhere patients get care. In pharmacies, they’re printed and placed on counters or handed out during consultations. In clinics, they’re pinned to bulletin boards in waiting rooms. In hospitals, they’re embedded in electronic health records. Epic Systems added FDA generics infographics to its patient portal in late 2022. Within six months, there were 450,000 views. Primary care doctors use them during visits. Pharmacists keep them on hand for last-minute questions. Community health workers hand them out at food banks and senior centers. One rural clinic in Oregon started printing them on half-sheets of paper and taping them to prescription bags. Patient questions about generics dropped by 52% in three months. Even insurance plans are using them. The Pharmaceutical Care Management Association now recommends that health plans provide “visually engaging educational materials about generic medications” to members. Some Medicare Advantage plans now include these infographics in welcome packets. Implementation is simple. No special software. No training required. Just download the PDF, print it, or link to it. The FDA even provides ready-to-use social media posts and information cards to help spread the word.What’s Missing-and What’s Coming Next
These infographics are powerful, but they’re not perfect. Some experts, like Dr. Aaron Kesselheim from Harvard, point out a blind spot: narrow therapeutic index drugs. Medications like warfarin, levothyroxine, or phenytoin require very precise dosing. Small differences in absorption can matter. Current infographics don’t clearly signal these exceptions. Patients might assume all generics are equally interchangeable-even when they’re not. The Institute for Safe Medication Practices (ISMP) recommends adding visual icons for drugs that require pharmacist notification during substitution. Right now, that’s only mentioned in small text. There’s also a gap in equity. While 34.7% of African American and 28.3% of Hispanic patients express higher concerns about generic quality, only one FDA infographic-“Generic Drugs and Health Equity Handout”-directly addresses this. Most materials assume all patients have the same level of trust, access, or prior experience. The future is brighter. In March 2023, the FDA released Version 2.0 of its core infographic, now showing $313 billion in annual savings from generics. In January 2023, GTMRx launched interactive digital versions where patients can input their meds and get personalized explanations. Even more exciting? The FDA is testing augmented reality. A prototype lets patients scan a pill bottle with their phone and see a 3D model of the active ingredient-brand and generic side by side-dissolving in real time. It’s still in testing, but early feedback is strong.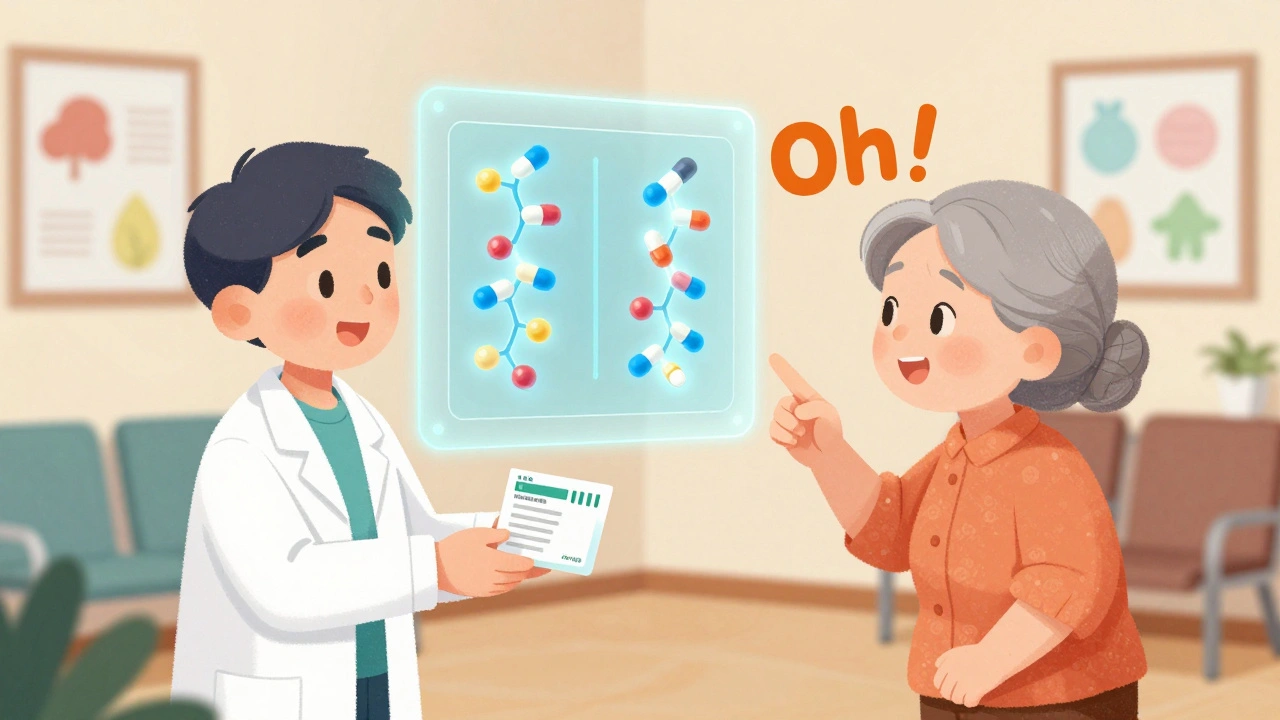
How to Use These Tools in Real Life
You don’t need to be a doctor or pharmacist to use these infographics. Here’s how anyone can benefit:- For patients: Download the FDA’s “What Makes a Generic the Same as a Brand-Name Drug?” infographic. Keep it on your phone or print it. Bring it to your next appointment. Ask your provider: “Does this match what’s shown here?”
- For caregivers: Use the infographic to explain to an elderly parent why their new pill looks different but works the same. Point to the molecular diagram. Say: “It’s the same medicine, just made by a different company.”
- For community health workers: Add the infographic to your health fair packets. Use it as a conversation starter. “Have you ever been told your medicine changed? Let me show you why that’s okay.”
- For pharmacists: Print copies and place them near the pickup counter. When a patient hesitates, say: “I have a picture that explains this. Can I show you?”
Final Thought: Knowledge Is the Real Generic
Generics save money. But they only work if people take them. And people only take them when they understand them. Infographics about generics aren’t just pretty pictures. They’re bridges between science and trust. They turn fear into clarity. They turn confusion into confidence. And they help patients make smarter, safer choices-without spending a dime more.Every time someone reads one of these visuals and says, “Oh, so it’s the same?”-that’s not just education. That’s healthcare equity in action.
Are generic drugs really as effective as brand-name drugs?
Yes. The FDA requires generic drugs to have the same active ingredient, strength, dosage form, and route of administration as the brand-name version. They must also meet the same strict standards for purity, stability, and performance. Bioequivalence testing proves they work the same way in the body. In fact, 90% of prescriptions in the U.S. are for generics because they’re proven safe and effective.
Why do generic pills look different from brand-name ones?
By law, generic drugs can’t look exactly like the brand-name version. That’s to avoid trademark infringement. So manufacturers change the color, shape, size, or markings-but not the active ingredient. The differences are only in inactive ingredients like dyes or fillers, which don’t affect how the medicine works. Infographics show this visually so patients understand it’s not a different drug.
Can I trust generics if they’re made in other countries?
Yes. The FDA inspects all manufacturing facilities-whether they’re in the U.S., India, China, or elsewhere. Every facility must meet the same quality standards. The FDA conducts over 4,000 inspections annually, and 99% of imported generic drugs pass inspection. Where a drug is made doesn’t change its safety or effectiveness.
Do generic drugs have the same side effects as brand-name drugs?
Yes. Since the active ingredient is identical, side effects are the same. Some patients report feeling different when switching, but that’s often due to changes in inactive ingredients-like a new coating that affects how quickly the pill dissolves. These changes rarely cause real problems, but if you notice new or worsening side effects, talk to your doctor or pharmacist.
Are there any drugs where generics aren’t recommended?
For most drugs, generics are safe and effective. But for a small group called narrow therapeutic index drugs-like warfarin, levothyroxine, or phenytoine-small differences in absorption can matter. These require close monitoring. While generics are still approved and used, doctors may choose to stick with one brand if a patient is stable on it. Infographics don’t always highlight this nuance, so always ask your provider if your medication falls into this category.
Where can I find these infographics?
The FDA offers all their generic drug infographics for free on their website at fda.gov/drugs/generic-drugs. You can download them as PDFs, print them, or link to them. They’re also available in Spanish. Some pharmacies and clinics also have printed copies available. No registration or payment is required.





11 Comments
Myson Jones
Just saw this at my pharmacy last week. Printed and laminated. My grandma asked why her new pill was blue instead of white. Showed her the infographic. She said, 'Oh, so it's the same medicine? I thought they were cutting corners.' Took me 30 seconds. No more confusion. That's the power of visuals.
Albert Essel
The FDA's approach here is a masterclass in science communication. By grounding every visual in bioequivalence data-dissolution curves, molecular structures, manufacturing checkpoints-they turn abstract regulatory standards into tangible, digestible truths. This isn’t just education; it’s trust engineering. And the WCAG compliance? Non-negotiable. Health equity isn’t a buzzword when your materials are accessible to someone with low vision or limited literacy.
Charles Moore
I’ve used these in my community health work in rural Ireland. We printed them on recycled paper, folded them into little booklets, and handed them out at the local clinic. One elderly man, who’d been refusing his generic blood pressure med for years, finally took it after seeing the side-by-side diagrams. He said, 'I thought they were just cheap knock-offs.' Now he’s saving €120 a month. These graphics don’t just inform-they heal.
Gavin Boyne
Let’s be real-pharma companies spend billions on ads telling you your brand-name drug is ‘special.’ Meanwhile, the FDA gives away free infographics that prove it’s all marketing BS. And people still don’t trust generics? Shocking. But also predictable. We live in a world where a $200 pill feels more ‘legit’ than a $4 one, even if they’re chemically identical. The real generic isn’t the drug-it’s the truth. And truth doesn’t come with a logo.
Rashi Taliyan
OMG I JUST HAD THIS EXPERIENCE!! My aunt in Mumbai was terrified to switch to generic levothyroxine because the pill was smaller and yellow. She cried. Said she felt like she was taking ‘fake medicine.’ I sent her the FDA infographic in Hindi-she showed it to her doctor, who then printed it and taped it to her prescription bottle. She’s now been on it for 3 months and says she feels ‘lighter.’ I cried too. This is why we need more of this. 🙏❤️
Kara Bysterbusch
The elegance of these infographics lies in their restraint. No hyperbolic claims. No emotive language. Just clean, data-driven visuals that speak louder than any physician’s spiel. The fact that comprehension scores outperform clinical consultations is not an accident-it’s design excellence. And the inclusion of Spanish versions? That’s not just translation-it’s cultural reciprocity. This is what public health should look like: dignified, deliberate, and deeply human.
Rashmin Patel
Okay but can we talk about how these infographics are basically the superhero version of patient education? 🦸♀️💥 I mean, they’re free, they’re multilingual, they’re ADA-compliant, they’re backed by science, and they actually WORK. I showed one to my cousin who’s on warfarin-he was freaking out because his new pills were round instead of oval. I pointed to the ‘narrow therapeutic index’ note and he was like, ‘Wait, so I need to stick with one brand?’ Exactly. And now he’s asking his pharmacist to label his bottle. This isn’t just info-it’s life-saving design. Also, can we make these into TikTok videos? I’d watch the hell out of a 60-second dissolving pill animation.
sagar bhute
Stop pretending this is some revolutionary breakthrough. The FDA’s infographics are just PR fluff wrapped in colorful charts. The real issue? Big Pharma controls the narrative. They pay doctors to push brand-name drugs. They lobby against generic substitution laws. And now they’re letting the FDA hand out pretty pictures to distract people from the fact that most generics are made in factories with zero oversight. This isn’t education-it’s a smokescreen.
Cindy Lopez
Typo in the post: 'phenytoine' should be 'phenytoin'. Also, the infographic doesn't mention that some generics have different bioavailability in fasted vs fed states. Minor, but still.
James Kerr
My pharmacist gave me one of these last week. I thought it was a flyer for a new soda. Turned out it was my blood pressure med. Now I keep it on my fridge next to the grocery list. Best thing I’ve seen all year. 🤙
Archie singh
Infographics? Really? You’re telling me the solution to decades of pharmaceutical misinformation is a PDF? The FDA’s entire credibility is built on regulatory rigor-not cartoon molecules. This is the equivalent of handing out a flowchart to explain quantum physics. It’s not that it’s wrong-it’s that it’s dangerously reductive. Patients need experts, not clipart.