When a drug’s patent runs out, something powerful happens: prices don’t just dip-they plummet. For patients paying hundreds a month for brand-name medication, that moment can mean the difference between affording treatment and skipping doses. This isn’t theory. It’s happening right now, with real people saving hundreds or even thousands each year the moment generics hit the market. But not all patent expirations are equal. Some drugs see near-instant price crashes. Others? They stay expensive for years-even after the patent technically expires.
How Patent Expiration Triggers a Price Crash
Patents give drugmakers a legal monopoly, usually for 20 years from the date of filing. That’s when they can charge whatever they want-no competition, no pressure. The average cost for a brand-name drug in the U.S. can be 10 to 20 times higher than what it costs to produce. But once that patent expires, the rules change. Any company can apply to make a generic version. And they do. Fast.
The first generic to enter the market usually cuts the price by 15% to 20%. That’s a start. But the real drop comes when the second, third, and fifth generics arrive. Each new player adds pressure. By the time 10 or more companies are selling the same drug, prices often fall 80% to 90%. The FDA has seen this pattern over and over. In 2023 alone, 870 generic drugs were approved-up 12% from the year before. Many of those were for drugs that had just lost patent protection.
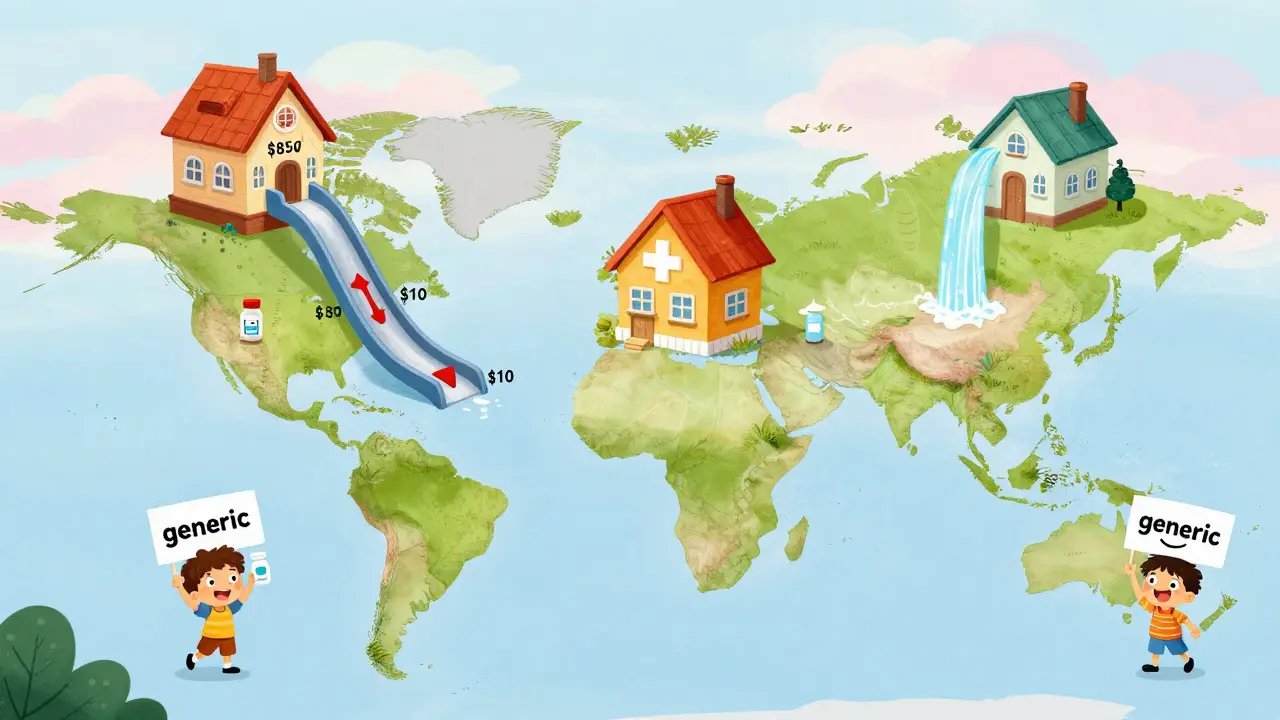
Take Eliquis, the blood thinner. Its patent expired in 2020. Before that, patients paid around $850 a month. Within a year, generics were available for under $10. Same active ingredient. Same effectiveness. Just a fraction of the cost. That’s not a fluke. It’s the rule.
Why Some Drugs Stay Expensive After Patent Expiry
Not every drug follows the script. Some stay expensive for years after their patent expires-not because they can’t be copied, but because the original maker won’t let them.
Take Humira, the rheumatoid arthritis drug. Its core patent expired in 2016. But AbbVie filed over 130 secondary patents on minor changes-delivery devices, dosing schedules, packaging. Each one delayed generic entry. By the time the first biosimilar, Amjevita, arrived in January 2023, Humira had already made over $200 billion in revenue. Even then, prices didn’t immediately drop. Why? Because AbbVie signed secret deals with insurers and pharmacy benefit managers. They offered deep rebates-so long as Humira stayed on the preferred list. The biosimilars? They were pushed to the back. Patients didn’t see savings until much later.
This trick is called “patent thickets.” And it’s everywhere. A 2025 report from I-MAK found that the average blockbuster drug accumulates 10 to 15 secondary patents. These aren’t new medicines. They’re tweaks. A new tablet shape. A different coating. A slightly adjusted dose. But under current rules, each one can add years of monopoly. For semaglutide (Ozempic, Wegovy), over 140 patents have been filed. The original patent expires in 2026. But experts say real competition won’t arrive until 2030 or later.

Price Drops Vary Wildly by Country
What happens after a patent expires isn’t the same everywhere. In the U.S., prices fall hard and fast-on average 82% over eight years. But in Switzerland? Only 18%. Why? Because Switzerland uses a system where the government sets a maximum price for drugs, regardless of competition. In Germany and the UK, prices drop 58% and 60% respectively, thanks to centralized negotiations and strict price controls.
Australia’s system is more like the U.S.-but with faster generic entry. After patent expiration, generics typically arrive within 12 months. That’s why Australia saw an 8-year price drop of 64%, one of the steepest in the study. Canada? 48%. Japan? 42%. The U.S. isn’t the fastest, but it’s the deepest. Why? Because it has the largest market, the most generic manufacturers, and the most aggressive price competition.
But here’s the catch: even in the U.S., insurance formularies can block access. If your insurer doesn’t list the generic as preferred, you might still pay full price. Some patients don’t even know a cheaper version exists. A 2023 Kaiser Family Foundation survey found that 22% of insured adults had their out-of-pocket costs delayed because their plan didn’t switch to the generic right away.
Biologics Are a Different Battle
Not all drugs are made the same. Traditional pills-small molecules-are easy to copy. The chemistry is simple. But biologics? These are complex proteins made from living cells. Think Humira, Enbrel, or insulin. You can’t just reverse-engineer them. That’s why they’re called “biosimilars,” not generics.
Getting approval for a biosimilar takes 7 to 10 years and costs $2 million to $5 million per product. That’s why they’re slower to arrive. The first biosimilar for Humira didn’t launch until 2023-seven years after the patent expired. Even now, only about 45% of the market has shifted to biosimilars in Europe. In the U.S., it’s even lower. The European Medicines Agency is pushing to get that number to 70% within three years of patent expiry. The U.S. is still catching up.
And here’s another twist: some biosimilars are priced almost the same as the original. Why? Because manufacturers still use rebates, discounts, and contracts to lock in hospitals and insurers. The price on the shelf might look low-but the real cost to the system hasn’t changed.
Who Benefits-and Who Gets Left Behind
The winners? Patients. Medicare. Medicaid. Employers paying for health plans. A 2023 Congressional Budget Office projection says generic and biosimilar competition will save the U.S. healthcare system $1.7 trillion over the next decade. That’s money that goes back into care, not corporate profits.
But the losers? Often, the patients who don’t know what’s happening. Or those whose doctors don’t prescribe the generic. Or those stuck on plans that delay substitution. A Reddit user in February 2024 wrote: “I switched from Eliquis to generic. My copay went from $400 to $15. I cried.” That’s the good story. But others report confusion. “I was told the biosimilar was the same. Then I got a bill for $600.” Why? Because the insurer hadn’t updated their formulary.
Pharmacists can substitute generics automatically in 49 states-but not always with biologics. Some states require a doctor’s approval. Others don’t allow substitution at all. That patchwork of rules means your savings depend on where you live.
What’s Next? The Fight Over Patent Abuse
Regulators are starting to push back. In 2023, the U.S. Patent Office cracked down on “patent thickets,” rejecting dozens of secondary patents that added no real innovation. The European Commission proposed limits on supplementary protection certificates-those are the legal tools that extend patents beyond 20 years. And the Inflation Reduction Act now lets Medicare negotiate prices for some drugs, which forces manufacturers to choose: lower prices now, or risk being left out of the program entirely.
But the system is still rigged in favor of the big players. The average blockbuster drug now makes 70% of its lifetime revenue after the patent expires-thanks to secondary patents, reformulations, and rebates. That’s not innovation. That’s delay.
For patients, the message is simple: when your drug’s patent expires, ask your doctor or pharmacist: “Is there a generic?” Don’t assume the brand is still the best option. Check your insurance formulary. Ask if you can switch. In most cases, the generic is just as good-and a fraction of the price.
Patent expiration isn’t magic. It’s competition. And competition works. But only if the system lets it.
How soon do drug prices drop after a patent expires?
The first generic usually arrives within 6 to 12 months after patent expiration in the U.S., and prices begin to drop immediately. The biggest price reductions happen within 1 to 3 years, especially when 5 or more generic manufacturers enter the market. In countries like Australia and the UK, generics arrive faster, so prices fall sooner. In the U.S., delays from patent lawsuits or complex regulations can push back meaningful savings by 1 to 2 years.
Why do some drugs stay expensive even after generics are available?
The original drugmaker often uses strategies like secret rebates, formulary restrictions, and contractual agreements with insurers to keep their brand on the preferred list. Even if a cheaper generic exists, if your insurance plan doesn’t cover it-or charges a high copay-you’ll still pay more. Some companies also delay biosimilar entry by filing dozens of secondary patents, a tactic known as “patent thickets.”
Are generic drugs as effective as brand-name drugs?
Yes. The FDA requires generics to have the same active ingredient, strength, dosage form, and route of administration as the brand-name drug. They must also meet strict bioequivalence standards-meaning they work the same way in the body. Studies show generics are just as safe and effective. The only differences are inactive ingredients (like fillers or dyes), which rarely affect performance.
What’s the difference between a generic and a biosimilar?
Generics are exact copies of small-molecule drugs made from chemicals. Biosimilars are highly similar versions of complex biologic drugs made from living cells. While generics are chemically identical to their brand-name counterparts, biosimilars can have minor differences due to their complex production process. Both are rigorously tested for safety and effectiveness, but biosimilars take longer and cost more to develop, which delays market entry.
Can I ask my pharmacist to switch me to a generic?
In most U.S. states, yes-unless your doctor specifically writes “dispense as written” on the prescription. Pharmacists are allowed to substitute approved generics automatically. For biologics, rules vary by state. Always ask your pharmacist if a cheaper option is available. You might be surprised how much you can save.
Do insurance plans always cover generics?
Not always. Some plans still favor brand-name drugs by placing generics in higher cost tiers or requiring prior authorization. Others delay switching until the next plan year. Check your formulary or call your insurer to confirm coverage. If a generic isn’t covered, ask your doctor to appeal or request a prior authorization.




8 Comments
Brett Pouser
Man, I saw this firsthand when my dad switched from Humira to the biosimilar. His copay dropped from $450 to $20. He didn’t even notice a difference in how he felt. That’s the thing nobody talks about - the system’s rigged, but the medicine? Still works. Just cheaper.
And yeah, I know some folks get scared of generics. But if the FDA says it’s good enough, I’ll take it. No need to pay for brand-name marketing when the pill’s the same.
Simon Critchley
Patent thickets? More like patent *tangles* - a legal spiderweb designed by Big Pharma’s in-house lawyers to strangle competition. The original 20-year monopoly? Fair. But when you file 140 secondary patents on *dye color* and *tablet shape*, you’re not innovating - you’re gaming the system.
And don’t get me started on rebate schemes. Insurers are complicit. They take the kickback, patients pay the price. It’s not capitalism. It’s feudalism with a pharmacy counter.
Karianne Jackson
I cried when my Eliquis generic came in. $400 to $15. I didn’t even know it was possible. My doctor never told me. My pharmacist didn’t say anything. I just got a bill one day and almost passed out. Why is this so hard? Why don’t people just TELL US?
Tom Forwood
So here’s the thing - generics aren’t just cheaper, they’re *faster*. I work in a clinic, and we’ve seen patients stop skipping doses once the price dropped. One lady told me she was using half her pills because she couldn’t afford the rest. Then generics hit. She said she finally slept through the night.
And yeah, biosimilars are trickier. Took forever for Humira alternatives. But now? We’re seeing 60% of new scripts go to biosimilars. Slow, but it’s moving.
Pro tip: Always ask your pharmacist. They know way more than your doc sometimes. And if they say ‘no generic yet,’ ask why. Chances are, it’s not science - it’s a contract.
Chelsea Cook
Ohhh so THAT’S why my insurance kept denying the generic? Because they were getting a kickback from AbbVie? Wow. Just wow.
So let me get this straight - we’re paying extra so the drug company can bribe our insurer to keep the $800 pill on the list? And we’re supposed to be grateful?
Y’all. This isn’t healthcare. It’s a reality show called ‘Who Can Outsmart the System?’ And we’re all just extras in the background, holding our breath while our wallets cry.
John Sonnenberg
Let me be crystal clear: The FDA doesn’t approve generics because they’re ‘good enough.’ They approve them because they’re IDENTICAL. The same active ingredient. The same dose. The same bioavailability. The only difference? The label. The packaging. The company name.
And yet people still think brand-name = better. That’s not science. That’s advertising. That’s fear. That’s corporate brainwashing. And it’s killing people - slowly, quietly, one skipped dose at a time.
Jessica Klaar
I just want to say - thank you for writing this. I’ve been on a biologic for 8 years. I’ve seen the price go from $3,000 to $1,200 after the first biosimilar. Then down to $300. Now it’s $20. I didn’t realize how much of a miracle that was until I started reading about how long it took - and how many barriers they put up.
My pharmacist finally told me about the switch last year. I wish someone had told me sooner. I wish more people knew this wasn’t magic. It was competition. And competition? It works. If you let it.
Also - if your doctor doesn’t mention generics, ask. Don’t wait. You’re not being difficult. You’re being smart.
Kathryn Lenn
Let’s be real - this whole ‘generic savings’ narrative is a distraction. The real story? The government let Big Pharma write the rules. Patent extensions? Rebate systems? Formulary lock-ins? All designed to keep profits flowing.
And now they want us to cheer because we’re paying $20 instead of $800? That’s not a win. That’s a downgrade from robbery to petty theft.
They’re not lowering prices because they care. They’re lowering them because they had to. And when the next blockbuster comes along? Same playbook. Same loopholes. Same silence from regulators.
Don’t get fooled. This isn’t progress. It’s damage control. And we’re still paying for it - just in different ways.