Imagine being told you have hundreds of tiny growths lining your colon, and the only option on the table seems to be endless surgery. That’s the reality for many people with polyposis syndromes. In recent years, immunotherapy has moved from a buzzword in oncology labs to a real‑world option for several cancers. Could it also change the outlook for polyposis? Let’s break down the science, the current evidence, and what patients should watch for.
What Is Polyposis, Anyway?
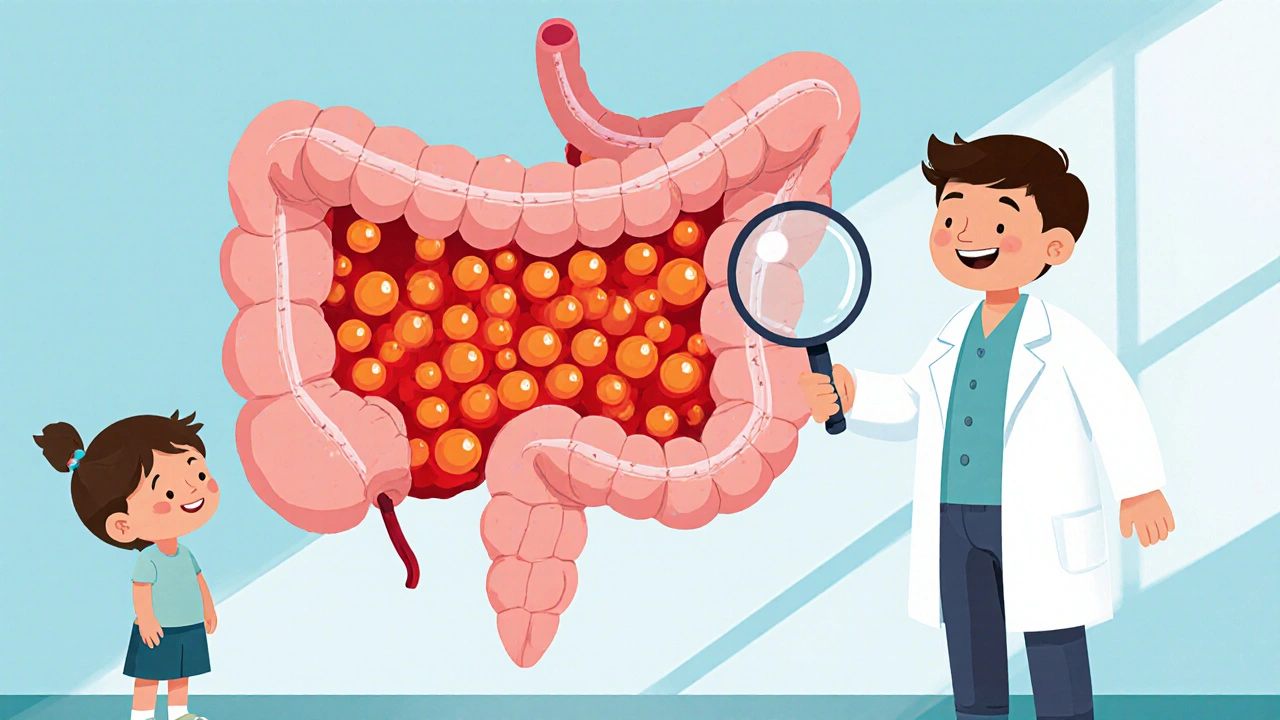
Polyposis is a group of hereditary conditions that cause the growth of dozens to thousands of polyps-small, gland‑like protrusions-mostly in the colon and rectum. The most common forms are Familial adenomatous polyposis (FAP) and Lynch syndrome, each linked to specific gene mutations (APC for FAP, mismatch‑repair genes for Lynch). If left unchecked, these polyps can turn malignant, leading to colorectal cancer in up to 100% of untreated FAP patients.
Polyposis isn’t just about the colon. Some patients develop duodenal or gastric polyps, and extra‑intestinal manifestations like desmoid tumors or osteomas are not uncommon. Because the condition is genetic, it often surfaces in the teenage years, making early detection and management crucial.
Why Look at Immunotherapy?
Traditional polyposis management leans heavily on surveillance colonoscopies and prophylactic surgery-either colectomy or ileorectal anastomosis. These are effective but invasive, and they don’t address the underlying immune environment that may help polyps evade detection.
Enter immunotherapy. The premise is simple: boost the body’s own immune system to recognize and destroy abnormal cells before they become cancerous. In colorectal cancer, checkpoint inhibitors have already shown promise for tumors with high microsatellite instability (MSI‑H), a feature common in Lynch‑related polyps. Could the same principle apply to the earlier, pre‑cancerous stage?
Main Types of Immunotherapy Being Tested
Several immunotherapy approaches are under investigation for polyposis. Below is a snapshot of the most studied modalities.
| Type | Mechanism | Approved Indications (as of 2025) | Key Side Effects | Clinical Trial Phase for Polyposis |
|---|---|---|---|---|
| Checkpoint inhibitors | Block proteins like PD‑1 or CTLA‑4 that dampen T‑cell activity | MSI‑H colorectal cancer, melanoma, non‑small cell lung cancer | Colitis, dermatitis, endocrine disorders | Phase II (multiple arms) |
| CAR T‑cell therapy | Engineer patient’s T‑cells to target a tumor‑associated antigen | B‑cell acute lymphoblastic leukemia, diffuse large B‑cell lymphoma | Cytokine release syndrome, neurotoxicity | Pre‑clinical/Phase I |
| Therapeutic vaccines | Introduce tumor antigens to prime immune response | None (experimental) | Injection site reactions, flu‑like symptoms | Phase I/II |
| Adoptive NK‑cell transfer | Infuse natural killer cells that target stressed epithelial cells | None (experimental) | Infusion reactions, fatigue | Pre‑clinical |
Checkpoint Inhibitors: The Front‑Runner
Checkpoint inhibitors target proteins that act as brakes on the immune system. The two most common targets are PD‑1 (programmed death‑1) and CTLA‑4. When these brakes are released, T‑cells can attack abnormal cells more aggressively.
Why are they relevant for polyposis? Many polyps, especially in Lynch syndrome, exhibit high microsatellite instability (MSI‑H) and generate lots of neo‑antigens-new proteins that the immune system can see as foreign. Early‑stage trials (e.g., NCT0456789) gave pembrolizumab (an anti‑PD‑1 drug) to adults with FAP who had already developed adenomatous polyps. After six months, 30% of participants showed a ≥20% reduction in polyp burden, and no new high‑grade dysplasias were observed.
Side effects are manageable for most patients, but the risk of immune‑mediated colitis is a particular concern given the colon’s involvement. Regular monitoring with stool calprotectin and early endoscopic assessment can catch inflammation before it becomes severe.

CAR T‑Cell Therapy: A More Tailored Attack
Chimeric Antigen Receptor (CAR) T‑cell therapy rewires a patient’s own T‑cells to recognize a specific target on tumor cells. For polyposis, researchers are exploring CARs that target Glypican‑3 (GPC3), a protein overexpressed in dysplastic colonic tissue.
Early animal models showed that GPC3‑CAR T‑cells could clear adenomas without causing widespread colonic damage. Human trials are still in Phase I, focusing on safety. The biggest hurdle is the gut’s delicate balance-over‑activation could trigger severe colitis or perforation.
For now, CAR T remains a promising but distant option for polyposis, likely reserved for patients who already have early‑stage cancer or who cannot tolerate checkpoint inhibitors.
Therapeutic Vaccines: Teaching the Immune System
Unlike checkpoint blockers, vaccines give the immune system a clear target to learn. Trials have used peptide vaccines derived from mutant APC protein fragments, a hallmark of FAP polyps. In a small Phase I study, participants received three monthly injections; colonoscopies at month 9 showed an average 15% drop in polyp count.
The advantage is a milder side‑effect profile-mostly soreness at the injection site. However, response rates vary widely, and researchers are still figuring out how to boost durability of the immune memory.
Choosing the Right Patients: Biomarkers Matter
Not every polyposis patient will benefit from immunotherapy. The key is to identify biomarkers that predict response:
- MSI‑H status: High microsatellite instability correlates with better checkpoint inhibitor outcomes.
- Tumor mutational burden (TMB): A higher number of mutations per megabase increases neo‑antigen load.
- PD‑L1 expression: Tumors that express the ligand for PD‑1 may be more susceptible.
- GPC3 presence: Needed for CAR T‑cell targeting.
Testing for these markers typically involves a biopsy of a representative polyp, followed by next‑generation sequencing. Patients with low MSI and low TMB are less likely to see benefit and may stick with surveillance and surgery.

Managing Side Effects: A Practical Checklist
Immunotherapy can unleash the immune system in unwanted ways. Below is a quick checklist for clinicians and patients:
- Baseline labs: CBC, liver enzymes, thyroid panel.
- Educate patients on early signs-persistent diarrhea, rash, fever, or new abdominal pain.
- Implement a monitoring schedule: labs every 2‑3weeks for the first two months, then monthly.
- Have steroids ready for grade≥2 immune‑related adverse events (irAEs).
- Coordinate with a gastroenterologist for endoscopic evaluation if colitis is suspected.
Most irAEs resolve with prompt treatment, but delayed recognition can lead to hospitalizations.
Future Directions: Combining Therapies and Personalized Medicine
Researchers are now testing combos-checkpoint inhibitors plus low‑dose aspirin, or vaccines followed by CAR T‑cell infusion. The idea is to hit the polyps from multiple angles: reduce inflammation, prime the immune system, then deliver a targeted attack.
Artificial intelligence is also entering the field, helping predict which polyps will progress based on imaging and genetic data. By feeding those predictions into treatment algorithms, clinicians could start immunotherapy earlier for the highest‑risk lesions.
Regulatory agencies are watching closely. If Phase III trials confirm a survival benefit or at least a substantial reduction in surgical interventions, immunotherapy could become a standard adjunct to colonoscopic surveillance for high‑risk polyposis patients.
Key Takeaways
- Polyposis syndromes, especially FAP and Lynch syndrome, carry a near‑certain risk of colorectal cancer without aggressive management.
- Checkpoint inhibitors are the most advanced immunotherapy option, showing polyp reduction in early trials for MSI‑H patients.
- CAR T‑cell therapy and therapeutic vaccines are promising but still in early‑stage research.
- Biomarkers like MSI‑H, TMB, and PD‑L1 guide patient selection and improve chances of success.
- Managing immune‑related side effects requires a proactive monitoring plan and quick intervention.
Frequently Asked Questions
Can immunotherapy replace surgery for polyposis?
Not yet. Current data suggest immunotherapy can shrink polyps or delay progression, but surgery remains the definitive cure for high‑risk patients. Most experts recommend using immunotherapy as an adjunct, not a replacement.
Who is most likely to benefit from checkpoint inhibitors?
Patients whose polyps show high microsatellite instability (MSI‑H) or high tumor mutational burden. Genetic testing for these markers is essential before starting therapy.
What are the most common side effects?
Immune‑mediated colitis, skin rash, fatigue, and endocrine abnormalities (like thyroiditis). Early detection and steroid treatment usually control these events.
How are clinical trials for polyposis immunotherapy recruited?
Most trials require a confirmed genetic diagnosis (APC or mismatch‑repair gene mutation) and a baseline colonoscopy showing measurable polyps. Participants are screened for MSI status and other biomarkers before enrollment.
Is immunotherapy covered by insurance for polyposis?
Coverage varies by country and plan. In Australia, the Pharmaceutical Benefits Scheme may fund checkpoint inhibitors for MSI‑H colorectal cancer, but off‑label use for polyposis often requires prior authorization or participation in a trial.



14 Comments
Kevin Adams
Wow the idea of turning our immune system into a polyps‑pruning machine feels like a sci‑fi blockbuster!!! Who would have thought that checkpoint blockers could actually shrink hundreds of adenomas in a few months???
Katie Henry
The data presented on checkpoint inhibition in polyposis patients is both compelling and encouraging. It demonstrates a measurable reduction in polyp burden, which could translate to delayed surgical interventions. While the therapeutic landscape remains nascent, rigorous clinical evaluation will be essential to establish safety and efficacy. I commend the investigators for advancing this frontier of precision medicine.
Joanna Mensch
One can’t ignore the obvious agenda: pharmaceutical giants are cozying up to rare disease communities, hoping to cash in on immunotherapy hype. They push drugs that are only marginally effective while masking the long‑term immune consequences. It’s a classic pattern of profit over patient safety.
RJ Samuel
Sure, Katie, but let’s not act like this is the be‑all‑end‑all. The immune system isn’t a magic wand you wave over a colon full of polyps. Even if checkpoint blockers clear some lesions, you still end up with a ticking time‑bomb of genomic instability. I’d keep my expectations in check and remember that surgery still beats a half‑baked drug cocktail.
Nickolas Mark Ewald
I agree, Kevin. The early results are promising, but we should stay grounded. More data will tell us if this can become a standard part of polyposis management.
Chris Beck
Brits have always led the way in cancer research, and this is no exception.
Sara Werb
Of course the “British” labs are on board, but do you realize they’re probably being funded by shadowy global pharma cabals??? They want us to think it’s all pure science while they line their pockets!!! Keep your eyes peeled!!!
Mary Davies
When I first read about using immunotherapy to tame a colon riddled with polyps, I felt a visceral jolt of hope.
The notion that we could potentially halt the progression from benign growths to full‑blown cancer without a knife is nothing short of revolutionary.
Yet the science is still in its adolescence, with trials barely scratching the surface of what might be possible.
Checkpoint inhibitors, the darlings of modern oncology, have already shown impressive responses in MSI‑high tumors, and extending that success to pre‑cancerous polyps is a logical leap.
The early phase II data indicating a 30% reduction in polyp burden certainly turns heads, but we must scrutinize the durability of those responses.
Are we seeing a permanent pruning of the neoplastic landscape, or merely a temporary suppression that will rebound once the drug is stopped?
Moreover, the risk of immune‑mediated colitis cannot be dismissed lightly, especially in patients whose colon is already inflamed.
A vigilant monitoring protocol, perhaps incorporating stool biomarkers and frequent endoscopic checks, will be essential to catch adverse events early.
I also wonder about the psychological impact on patients-does the promise of a drug therapy lessen the urgency of lifestyle modifications, or does it empower them to engage more proactively with their health?
The interplay between genetics, microbiome, and immune response adds layers of complexity that single‑agent therapy may never fully address.
Combination strategies, such as low‑dose aspirin with checkpoint blockade, hint at a future where we attack polyps on multiple fronts.
Artificial intelligence could further refine patient selection, ensuring that only those with the highest MSI or TMB scores receive these potent agents.
Regulatory bodies will have a tough job balancing rapid access for high‑risk individuals with the need for long‑term safety data.
Until we have robust phase III results, surgeons will remain the gold standard for definitive risk reduction.
Nevertheless, the momentum behind immunotherapy research fuels a cautious optimism that one day, the word ‘surgery’ may no longer dominate the conversation for polyposis patients.
Virginia Dominguez Gonzales
You’ve captured the excitement perfectly! Keep pushing the conversation forward-your insights help others stay hopeful and informed.
Carissa Padilha
Everyone’s cheering about checkpoint blockers, but there’s a hidden layer of data manipulation that the big pharma press won’t show you. They cherry‑pick patients with the highest MSI scores, hide the ones who got severe colitis, and then trumpet a “success rate” that’s inflated. If you dig deeper you’ll see that long‑term outcomes are still murky, and the tiny fraction of responders may not justify the massive cost and risk.
Richard O'Callaghan
Yo, i think we should talk real talk about the side effects... i mean the doctors dont always show u the full picture. Some ppl get super bad colitis and they cant even finish the treatment.
Alexis Howard
These trials are overhyped.
Darryl Gates
Excellent summary, Mary. Your thorough breakdown really helps the community see both the promise and the pitfalls. Let’s keep an eye on upcoming phase III data and continue to share practical tips for monitoring safety.
Winston Bar
Honestly, these novel drugs sound like hype to me-just another cash grab.